APPLICATIONS OF TECHNOLOGY:
- Developing therapeutics for the radiosensitization of cancer cells
- Assessing the efficacy of cancer treatments
- Cancer drug screening and development
- Research on the in vivo activity of DNA-PK
ADVANTAGES:
- Identifies two major DNA-PKcs autophosphorylation sites which are involved in DNA double strand break repair
- First technology to enable the in vivo monitoring of DNA-PK response to DNA damage
- Offers an antibody for drug screening and development
ABSTRACT:
David Chen, Ping-Chi Benjamin Chen, and Doug Chan have demonstrated that autophosphorylation of DNA-PKcs is an important event in the repair of DNA double strand breaks (DSBs) by nonhomologous end-joining. The Berkeley Lab researchers have identified two autophosphorylation sites on the extremely large DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and have generated antibodies that will enable the in vivo monitoring of DNA-PK response to DNA damage.
The newly discovered autophosphorylation sites are located at Threonine 2609 and Serine 2056. A mammalian cell line that expresses a mutation at Threonine 2609, preventing DNA-PKcs autophosphorylation at the site, showed an increase in cell radiosensitivity.
Ionizing radiation and cancer drugs inflict DSBs in order to kill cancer cells. The ability to intervene in autophosphorylation of T2609 or S2056 and hinder DBS repair, either through application of a drug or an antibody, would increase the radiation-induced killing of cancer cells. Therefore, identification of these critical autophosphorylation sites is a first and necessary step in advancing this line of cancer treatment.
“A cell lacking this kinase will become extremely radiation sensitive,” says David Chen. A drug that renders the two identified sites inert would “reduce a patient’s radiation dose and reduce their side effects.”
Dr. Chen and colleagues have generated antibodies that recognize Threonine 2609 and Serine 2056 but do not bind to the unphosphorylated DNA-PKcs protein or peptide. These antibodies identify areas where DSBs are being repaired and therefore can be used to monitor the effectiveness of treatments that target the DNA repair pathway of cancer cells. Before the Berkeley Lab phosphorylation-specific antibodies were identified, DNA-PK activity could not be monitored in vivo. Because there is an abundance of DNA-PK in a cell’s nucleus, it has been impossible to distinguish between the large DNA-PK background signal and DNA repair protein foci using immunofluorescence and currently available antibodies.
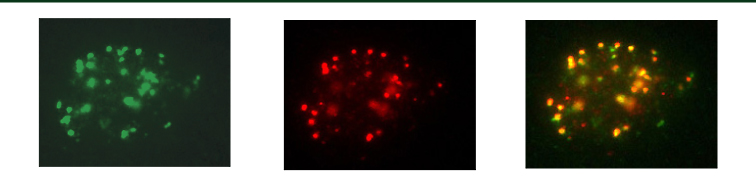
Left: gH2AX staining shows damaged DNA. Middle: pT2609 antibody staining shows phosphorylated DNA-PKcs. Right: Merging of the images on the left shows colocalization. Images of irradiated human primary fibroblasts provide the first evidence that DNA-PKcs is localized to sites of DSBs in the cell and is thus at the right location to play a direct role in DSB repair. The bright yellow spots show where the green and red foci are aligned.
STATUS:
- Issued Patent # 7,491,804. Available for licensing.
REFERENCE NUMBER: IB-1807