E.O. Lawrence Berkeley National Laboratory
APPLICATION OF TECHNOLOGY:
- Lithium metal polymer and lithium polymer batteries
- PEM fuel cells
- Electrochromic windows
- Electroseparations
ADVANTAGES:
- High lithium ion conductivity (10-5 S/cm at ambient temperatures)
- No concentration polarization, i.e. the transference number is one
- Clean grafting and cross-linking chemistry leaves no reactive residues
- Materials have uniform, reproducible mechanical properties and electrochemical stability
- Polymer backbone and cross-link density and flexibility may be adapted to tune the transport and mechanical properties
- Innovative solution to lithium mobility problems promises even higher conductivity
- Easy preparation
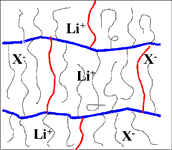
Cross-linked comb-branch structure. Heavy horizontal lines represent the comb-branch backbone, heavier vertical lines the cross-links and the lighter vertical lines the solvating ether side-chains. Anions are fixed to the side chains. Mechanical properties are a function of the backbone structure and cross-link density. Ionic mobility is a function of the solvating groups incorporated in the side chains. The molecular structures are infinitely variable in order to provide optimum properties for both bulk membranes and composite electrodes (MEAs).
ABSTRACT:
John Kerr and co-workers at Berkeley Lab have developed single-ion cross-linked comb-branched polymer electrolytes with high conductivity for use as membranes in lithium batteries, fuel cells, and electrochromic windows. Solid polymer electrolyte separators are used in lithium batteries instead of common organic solvents because (1) they are non-volatile, (2) they inhibit the growth of dendrites, the tiny metallic snowflake structures in lithium metal electrodes that lead to battery failure, and (3) they can be used in very thin films thereby improving the power performance of the battery and increasing the energy density.
Solid polymer electrolytes have been improved by the creation of single-ion polymer conductors. Single ion conductors, transference number of one, avoid the development of concentration gradients that result in low voltage upon discharge and irreparable damage on charge because the anion is immobilized by covalently connecting it to the polymer comb. Until now, lithium single ion polymer conductors have been plagued with low conductivity, reactivity to lithium, poor cathode compatibility, and mechanical stiffness that leads to poor processing properties. Kerrs new cross-linked polymer electrolytes based on trifluoromethylsulfonylmethide, sulfonate, and fluoroalkylsulfonate and imide anions overcome these limitations.
The controllable method of preparation results in a material that has uniformly excellent mechanical and ion transport properties that appear to be unaffected by the cross-linking density. This allows density to be varied to suit the application. The cross-linked materials achieve much higher lithium ion conductivities than other cross-linked polymers (10-5 S/cm at ambient temperatures) and yet also inhibit dendrite growth due to the mechanical properties. The side chains of the comb-branched structures are long enough to allow for maximum segmental motion so that the polymer can effectively penetrate between the electrode particles and adhere to electrode surfaces while maintaining the amorphous nature that facilitates high ion mobility. This overcomes many of the problems involved in the preparation of good composite electrode structures.
The capabilities, materials, and principles used for developing these polymer electrolytes for lithium batteries can be adapted to develop polymer films for fuel cells and electrochromic windows. Kerrs group is investigating the use of new proton solvating functions on comb branch polyether polyelectrolyte materials to provide water-free membranes that can operate at high temperatures for fuel cells.
STATUS:
PUBLICATION:
REFERENCE NUMBER: IB-1553, 1554