APPLICATIONS OF TECHNOLOGY:
- Binder for sulfur electrodes in lithium-sulfur (Li-S) rechargeable batteries
- Binder for negative and positive electrodes of lithium-ion batteries and advanced lithium-ion batteries
BENEFITS:
- The three-dimensional network structure of the dissolvable ionic crosslinked polymer (DICP) significantly enhances the mechanical integrity of the composite electrode for high loading electrode formation.
- The novel water-soluble binders enable the design of recyclable batteries.
BACKGROUND:
- Among the various possible candidates of electrode materials, sulfur offers several advantages (e.g. natural abundance, cost saving and superior theoretical capacity (1675 mAh g-1), energy density (2600 Wh kg-1)). However, the practical application of Li-S batteries is still limited by the following issues: (1) the dissolution and diffusion of polysulfide intermediates, (2) the poor electron and ionic conductivity of sulfur sulfides, and (3) large volume changes.
- Crosslinked polymer binders with different functional groups can significantly improve electrochemical performance. However, most of the crosslinked polymers are un-dissolvable once they are polymerized. Therefore, the highly valuable components in electrodes are unable to be recycled through a simple method.
TECHNOLOGY OVERVIEW:
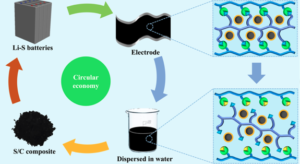
In order to reduce the overall cost of batteries considerably, strategies are needed to easily recycle the carbon hosts at their end-of-life. In addition, Li-S batteries, which have attracted a great amount of attention in recent years, are still hindered by suppressed delivered capacity and fast capacity fading during cycling. To address these issues, researchers at Berkeley Lab have invented a dissolvable ionic crosslinked polymer (DICP) as a binder for Li-S batteries.
The DICP has multi-functional bonding groups and was prepared via a pH-controlled interaction. Therefore, the crosslinked binder network can be readily dissociated in basic conditions, enabling valuable components to be recycled using environment-friendly processes. Additionally, re-fabricated Li-S cells were able to deliver the discharge capacity at a comparable level to fresh materials. Evidence from cell performance and characterizations such as in-situ X-ray adsorption spectroscopy (XAS), in situ UV-visible spectroscopy (UV-vis), X-ray photoelectron spectroscopy (XPS), and Density Functional Theory (DFT) calculation confirmed that the DICP is a more effective binder than its commercial counterpart on suppressing polysulfide dissolution in the electrolyte. For instance, the DICP binder enabled a high sulfur loading electrode of 5.5 mg cm-2 with an areal capacity of 6.39 mAh cm-2 while preserving stable cycling stability.
This study provides an eco-efficient and sustainable design to recycle electrode materials, supporting the development of the future circular economy (e.g. large scale energy storage systems). In addition, to the researchers’ knowledge, this is the first report of recyclable Li-S batteries. With the application of the robust binder, Li-S batteries could achieve excellent electrochemical performance.
DEVELOPMENT STAGE: Proven principle
PRINCIPAL INVESTIGATORS:
STATUS: Patent pending.
OPPORTUNITIES: Available for licensing or collaborative research.
SEE THESE OTHER BERKELEY LAB TECHNOLOGIES IN THIS FIELD:
Conductive Polymer Binders for Aqueous Processing of Battery Electrodes 2018-087