Electrocatalyst for CO2 to CO conversion with 99% selectivity and ~10% improvement in energy efficiency over competing catalysts, with minimal use of precious metals and at ambient temperature/pressure
APPLICATIONS OF TECHNOLOGY:
- This invention is best suited for companies looking to efficiently convert large CO2 waste streams into useful feedstock CO-using renewables:
- Fuels, concrete, methanol, polymers, and aggregates
- Pseudocapacitor energy storage
BENEFITS:
- Catalyst demonstrates substantial enhancement in CO production rate per catalyst surface area in comparison to previous technologies
- Achieves nearly unit CO selectivity (~99%) at much lower energy input compared to existing electrocatalysts
- Can be created using various metal particles and organic ligands, and can be applied in various electrochemical conditions
- Exhibits stable long-term catalytic performance
BACKGROUND:
A recent report] from SusChem has highlighted the major technical and economic challenges for an electrocatalyst to work. Specifically, the need to create ideal catalytic machineries for enzyme mimicry requires the precise configuration of multiple functional groups and mobile parts that dynamically respond to external stimuli. However, their manipulation has been limited by strategies that are restricted to ligands in a tethered configuration. The invention ultimately overcomes this issue by containing a multi-component catalytic pocket for high-specificity CO2 electrocatalysis.
TECHNOLOGY OVERVIEW:
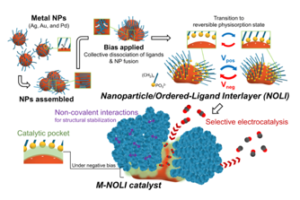
Researchers at Berkeley Lab led by Peidong Yang have developed a nanoparticle-ligand composite catalyst for selective CO2 electroconversion. Possessing a pseudocapacitive interlayer known as a nanoparticle/ordered-ligand interlayer (NOLI), the invention is the first demonstration of pseudocapacitance within this structural context.
The NOLI creates an active site configuration that enhances catalytic turnover by two orders and one order of magnitude against a pristine metal surface and small nanoparticle with tethered-ligands, respectively. The catalyst can be used in high-rate CO2 electrolysis reactors and demonstrated its nearly unit CO selectivity at commercially-relevant current densities (e.g., 98.1% at 400 mA/cm2), and further exhibited substantially high current density per catalyst mass loading (e.g., 2921 A/g).
The main advantages of this technology include not only the reduced energy input for selective CO2-to-CO electroconversion (i.e. improved energy efficiency), but also minimal costs of materials and for product separation. Notably, it achieves ~10% improvement in energy efficiency over competing catalysts while using 5-20x fewer precious metals and at ambient temperature and pressure.
In addition, this invention is the first demonstration of pseudocapacitance at a metal-organic heterostructure where cations are inserted. Companies developing materials for high-rate electrochemical energy storage devices such as pseudocapacitors can potentially use this invention as a new electrode material.
DEVELOPMENT STAGE: Proven principle
FOR MORE INFORMATION:
PRINCIPAL INVESTIGATORS:
STATUS: Patent pending.
OPPORTUNITIES: Available for licensing or collaborative research.
SEE THESE OTHER BERKELEY LAB TECHNOLOGIES IN THIS FIELD:
Nanoparticle Catalysts for Carbon Dioxide Reduction 2017-003