APPLICATIONS OF TECHNOLOGY:
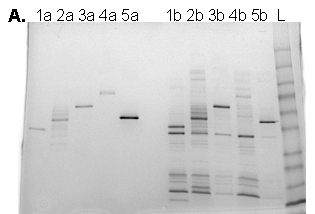
Purification of five different His-tagged proteins expressed in E. coli using the Berkeley Lab His-tag aptamer magnetic beads (1a-5a) or commercially available Ni-coated magnetic beads (1b-5b).
- Detection, purification, and capture of His-tagged and native proteins
- Surface Plasmon Resonance (SPR) technology
- Generation of aptamer-derived therapeutics
ADVANTAGES:
- Superior detection, purification, and capture of His-tagged and native proteins
- Less labor intensive, less expensive, and faster method of aptamer production
- Easily scalable for high-throughput production without the need for robotics
- Standardized for a diverse array of proteins
- Additional purification step negates the possibility of generating aptamers against contaminants
ABSTRACT:
Aptamer for His-tag
Sharon Doyle and co-workers at Berkeley Lab have developed an aptamer that is highly specific for the commonly used histidine tag. These qualities make the aptamer ideally suited for the capture, detection and affinity purification of His-tagged proteins.
The His-aptamer is likely to be of significant value for surface plasmon resonance (SPR) technology in protein chips and biosensors. SPR is currently underserved by sub-optimal His-antibodies used in detection. An additional advantage of using the Berkeley Lab aptamer in the SPR system is that it enables all proteins to be aligned in a specific orientation. Overall, the Berkeley Lab aptamer is anticipated to significantly improve quality and reproducibility in SPR applications.
The His-aptamer also enables superior purification of His-tagged recombinant proteins. Magnetic beads coated with the Berkeley Lab aptamer are able to purify diverse His-tagged proteins more effectively than Ni-coated magnetic beads (Figure 1).
An improved method for the generation of high affinity aptamers
The Berkeley Lab scientists have developed an improved method to generate aptamers that can be used against native proteins. This method will be of value in applications when a tag affects protein structure or function. It can be used alongside the His-tag aptamers for complementary parallel processing of a given protein.
The Berkeley Lab method generates aptamers against the protein portion of a His-tagged protein. These aptamers can then be used for the detection, purification, and capture of native proteins.
The improved method is less labor intensive, less expensive, easily scalable, and increases the utility of the aptamers in downstream applications. By using a generic tag, the protocol does not require standardization for different proteins. Moreover, an additional purification step also negates the possibility of raising aptamers against contaminants. The method has been adapted for use with a publicly available sequence that allows easy one-step cloning.
STATUS:
- Published patent application. Available for licensing and collaborative research.
FOR MORE INFORMATION:
REFERENCE NUMBER: IB-1929